More Information
Submitted: 22 June 2020 | Approved: 15 July 2020 | Published: 16 July 2020
How to cite this article: Aznar M, Tobella L, Rivera A. Premaxillary osteotomy in children with bilateral cleft lip and palate: Skeletal and dental changes. J Oral Health Craniofac Sci. 2020; 5: 011-016.
DOI: 10.29328/journal.johcs.1001032
Copyright License: © 2020 Aznar M, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Premaxilla osteotomy; Bilateral cleft lip and palate; Cephalometric analysis
Premaxillary osteotomy in children with bilateral cleft lip and palate: Skeletal and dental changes
Mireia Aznar Gomez1*, Lluisa Tobella Camps1 and Alejandro Rivera Baró2
1Adjunct of Orthodontic and Odontopediatric Service of Sant Joan de Déu University Hospital, Barcelona, Spain
2Chief of Orthodontic and Odontopediatric Service of Sant Joan de Déu University Hospital, Barcelona, Spain
*Address for Correspondence: Mireia Aznar Gomez, Adjunct of Orthodontic and Odontopediatric Service of Sant Joan de Déu University Hospital, Barcelona, Spain, Email: [email protected]
Purpose: To evaluate changes in children with bilateral cleft lip and palate (BCLP) who premaxillary osteotomy and secondary alveolar bone grafting as compared to children with BCLP who are not indicated for surgery, and to determine variables that differentiate patients who do or do not require osteotomy.
Material and methods: Twenty-four children with BCLP were included in the study: 12 who underwent osteotomy (intervention group) and 12 who had no surgery (control group). Radiographic and model values of the intervention group were compared before (T1) and after (T2) premaxillary osteotomy, and measurements were compared with those from the control group at T1.
Results: Convexity, ANB (point A-nasion-point B), and maxillary depth was more diminished at T2 in children in the intervention group. Point A, anterior nasal spine, and pogonion were retroposed after surgery, and the anterior spine was higher. At T2, the upper incisors were proinclinated and intruded, and overbite was improved.
Models revealed increased intermolar intercanine width as well as intrusion of upper incisor after surgery. Premaxilla and upper molars were more extruded, had a higher total maxillary height and increased extrusion of upper incisor in children who underwent osteotomy.
Conclusion: After surgery, children who undergo surgery have a premaxilla that is more normalized and more level with the occlusal plane, as well as improved dental inclination. Variables that differentiate children who require osteotomy from those who do not include more extrusion and protrusion of the premaxilla, and a greater extrusion of the upper incisors.
Cleft lip and palate (CLP) is defined as a fissure, or cleft, due to failure of fusion between the maxillary and frontonasal processes of the face during embryonic development. It is the most common congenital malformation in the maxillofacial area, with a variable incidence according to races, with an estimated 1 per 1,000 births for Caucasians. It occurs more frequently in males [1].
The etiology of cleft palate is multifactorial, with contributions from both genetic and environmental factors. The genetic contribution is estimated to be 20–50% and is currently considered to be the most relevant cause of cleft palate. Factors involved in this process include the platelet-derived growth factor C (PDGF-C), genes such as MSX1, the TGFB3 growth factor, RARalpha retinoic acid receptor, and the transcription factor ARNT2. The environmental contribution is between 20% – 25% and can include a combination of factors, such as environmental exposure, maternal diet, (prescription and non- prescription) drug use, and cigarette smoking. It has been shown that pre-pregnancy obesity, diabetes, drugs such as corticosteroids, and parental age are associated with an increased risk of cleft palate [2,3].
Facial morphogenesis begins towards the end of the fourth week of gestation, with the growth of 5 prominences or processes formed mainly by the first pair of pharyngeal arches which will surround the stomodeum, or primitive mouth. Specifically, these are the frontonasal process and the maxillary and mandibular processes, which are composed of mesenchyme surrounded by oral epithelium. These processes, comprising cells of the neural crest, proliferate and fuse to form the face. Defective fusion (either partial or complete) of the maxillary process with the medial nasal gives rise to unilateral or bilateral fissures, which can occur either anterior to the incisive foramen or due to a defect in the fusion of the palatal processes posterior to the incisive foramen [3].
CLPs were one of the first malformations described by ultrasound, allowing a diagnosis up to the 20th week of gestation. Three-dimensional ultrasound now allows imaging of all possible projections, which increases the possibility of earlier diagnosis for cleft lip and palate [4].
Defects vary from small microform clefts in the lip vermilion, lip, or nostril, to a complete unilateral or bilateral CLP. Fusion of the secondary palate progresses posteriorly from the incisive foramen, and its defects can vary, ranging from a simple bifid uvula or a simple cleft palate, to a complete cleft of the hard and soft palates or to submucous cleft palate [3].
CLPs are most frequently unilateral, with a higher incidence on the left side; these are followed in frequency by the palatal clefts, and finally bilateral and labial clefts (which have similar incidence rates).
About 73% of CLPs are isolated manifestations, while 17% are associated with other malformations, and 9%, with syndromes [1,2].
There can also be associated dental anomalies, including alterations in the number of teeth (64.2%), with dental agenesis having the highest incidence (44.3%), with the upper lateral incisor (33.8%) the most frequently associated on the side of the cleft (74%). Additional defects include supernumerary (19.8%), ectopic, or microdontic teeth [2-18].
These congenital problems cause difficulties in feeding, language development, hearing, and dental and facial development and are likewise associated with difficulties in communication and social integration.
The treatment of CLP is complex; it begins at birth and continues into adulthood, and it requires multidisciplinary participation of specialists, including speech therapists, psychologists, surgeons, orthodontists, and otolaryngologists [2,5].
Bilateral cleft and lip palate (BCLP) is one of the most complex craniofacial malformations. Protrusion and extrusion of premaxilla is a typical feature (1). Premaxillary protrusion is caused by the unrestricted growth of the anterior nasal septum and the vomero-premaxillary suture [7]. While management of repositioning the premaxilla is controversial, performing a premaxillary osteotomy is indicated for improving the function and aesthetics of the patient, correcting the overbite, aligning the maxillary arch, increasing the success of the closure of oronasal fistulas, and improving integration of alveolar bone grafts [7,9].
To determine the need the premaxilla osteotomy, a joint visit is made with the orthodontist and the maxillofacial surgeon, where the need to perform the surgery or not is decided. Surgery is indicated in cases that present a severe extrusion of the premaxilla, serious sagittal alterations, negative torque of the upper incisors, rotated premaxilla, and/or if it requires leveling the occlusal plane or reducing the space between the alveolar fissures.
In cases of a severe malpositioned premaxilla, the protocol in Sant Joan de Déu Hospital premaxillary osteotomy is performed a premaxillary osteotomy at 8 to 12 years of age, although in more severe cases, it is performed earlier. Prior to premaxillary osteotomy, an orthodontic maxillary expansion is performed with a Hyrax disyunctor or quad helix to improve surgical access to the fissure area and to be able to relocate the premaxilla.
Under general anesthesia and orotracheal intubation local anesthetic with epinephrine is infiltrated. Surgical approach is virtually planned according to the patient’s anatomy [18]. The first option is an endonasal approach [19] to avoid adding a scar in the vestibular oral mucosa. An ophthalmic or a number 15 scalpel blade is used to perform the incision, submucosal dissection with a Molt periosteal elevator is done and osteotomies are performed with a piezoelectric device (NSK Variosurg 3). Bony interferences are released and premaxilla is fixed to the surgical splint with metal ligatures to the braces. Hemostasis is checked and mucosa is sutured with 4/0 Monocryl.
After the premaxillary surgery, a surgical acrylic splint is placed on the teeth with bands and brackets for 2–3 months for stabilization. Subsequently, alveolar grafts from the iliac crest of the same patient are performed. These procedures are carried out in two distinct surgeries to increase the stability of the premaxilla, improve vascularization, and increase the success of the alveolar grafts. Once growth is completed, the patient is evaluated as a prospective candidate for orthognathic surgery.
This technique is performed only in patients with bilateral lip-palatal fissure, and is performed infrequently, although in cases that require it, it is very important to restore the function and aesthetics of the patient at an early age.
Not all patients with BCLP require premaxillary osteotomy; thus, analyzing the variables that can differentiate both groups is of high interest.
The objectives of this study were to evaluate skeletal and dental changes after premaxillary osteotomy in patients with BCLP and to compare them with those of a control group of patients with BCLP but who did not undergo osteotomy.
The sample comprised an intervention group of 12 patients with complete Bilateral Cleft Palate who were indicated to undergo premaxillary osteotomy in consecutive cases and a control group of 12 patients with BCLP who did not require osteotomy. Patients were treated at Hospital Sant Joan de Déu Barcelona (Spain) from 2011 to 2015.
We excluded from the study all syndromic patients or those with a lack of good radiographic records.
All cases were performed by the surgical team at the Sant Joan de Déu Hospital in Barcelona with the same technique above mentioned.
Prior to premaxillary osteotomy and maxillary expansion, the intervention group had a lateral cranial teleradiography (T1), and models of the maxillary arch (M1) were recorded at the onset and then again after premaxillary osteotomy (T2, M2).
In the control group, the 12 patients with BCLP who did not undergo osteotomy were selected before maxillary expansion. Patients of the same age and sex were selected for the control group as for the intervention group for T1 (case-case-control).
In the cephalometric analysis, the following variables were evaluated to assess the skeletal class and facial growth: convexity (CONVX), maxillary depth (MXD), facial axis (FA), SNA angle, and SNB angle. At the dental level, the inclination of the upper incisor with the palatal plane (INCSUP-FH), dental plane (INCSUP), the underbite (UNDER), and the overbite (OVER) were recorded.
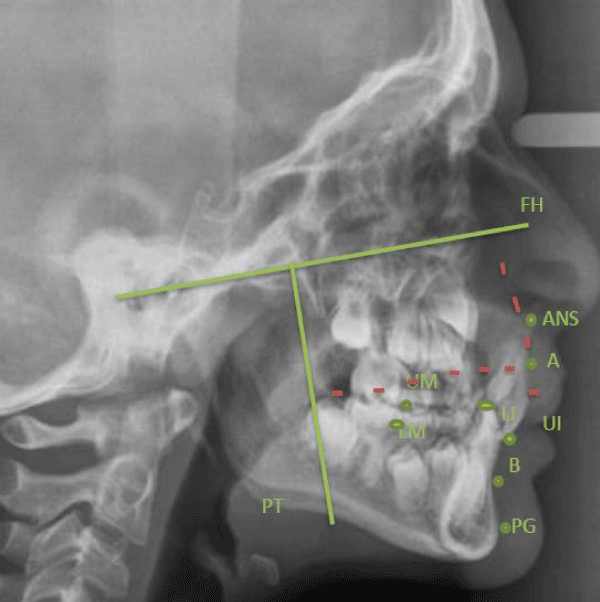
To evaluate the changes in vertical and horizontal directions after osteotomy, two reference planes were drawn: the Frankfurt horizontal (FH) plane and the plane perpendicular to this plane passing through the PT point. The distances of the following points to these two reference planes were measured: point A, point B, anterior nasal spine (ANS), incisal edge of the upper incisor (UI) and lower incisor (LI), mesial cusp of the upper molar (UM) and the lower molar (LM), and pogonion (PG) (Figure 1).
Figure 1: Distances from the following points to the two reference planes: Frankfurt horizontal (FH) plane and vertical plane perpendicular to this plane passing through point (PT): point A, point B, anterior nasal spine (ANS), incisal edge of the upper incisor (UI) and lower incisor (LI), mesial cusp of the upper molar (UM) and the lower molar (LM), and pogonion (PG).
In the sample of 3d models it consisted of 8 patients because we had to delete 4 records for not being of good quality.
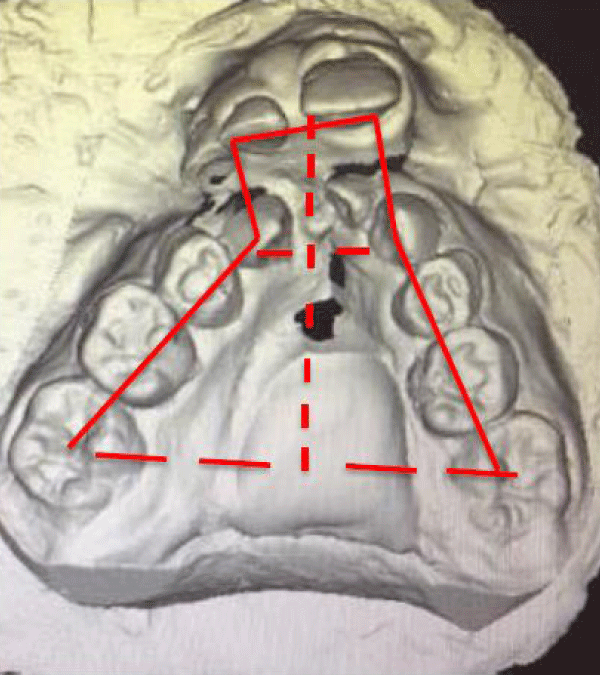
Regarding therefor model analysis, the models of the upper arch were scanned in the control group and the intervention group (M1–M2) before the maxillary expansion phase using the Optical Reveng 2.0 Dental Biotech 3D Scanner. The following measurements were made with the 3D Nemocast Software: intermolar width (INTERM), Intercanine width (INTERC), arch perimeter (PERIM), anterior maxillary height (ANT MX H), total maxillary height total (TOTAL MX H), and extrusion of the upper incisor (EXTR UI) (Figure 2 ).
Figure 2: Measures evaluated in the 3D model: intermolar width, intercanine width, arch perimeter, anterior maxillary height, total maxillary width, and extrusion of the lower incisor.
Values were measured twice by the same operator at different times, and the average was taken to eliminate method error.
Descriptive statistics of the intervention group were recorded at T1 and T2 for the cephalometric analyses and arch dimension variables, and for the control group at the beginning of treatment.
The IBM SPSS statistical package was used to determine statistically significant differences. Further, in the intervention group, statistically significant differences were determined at T1 and T2 using the statistical test of Wilcoxon rank, and at T1 and the control group, with the Mann–Whitney U-test. A significance value of p < 0.05 was required.
Firstly, we carried out the comparative study group (before and after osteotomy to evaluate cephalometric changes and models after surgery and subsequently assess the variables that can differentiate surgical cases from non-surgical cases (study group before surgery-control group).
The intervention and the control groups each were comprised of 12 children, with 2 girls and 10 boys. Prior to premaxillary osteotomy and maxillary expansion, the intervention group had a mean age of 11.3 years; after premaxillary osteotomy (T2, M2), the patients had a mean age of 13.2 years. In the control group, the 12 patients with BCLP who did not undergo osteotomy were selected before maxillary expansion; patients of the same age and sex were selected as the intervention group for T1, with an average age of 11.3 years (case-case-control).
At the level of cephalometric analysis, the intragroup comparison (T1–T2 in study group) revealed a decrease in convexity (6.2°), ANB (4.2°), and maxillary depth (4.6°) following premaxillary osteotomy. Further, the premaxilla (11.9 mm) and pogonion (6.8 mm) were retroposed, and the nasal spine (2.8 mm) was higher in T2. In terms of dental aspects, the upper incisors (22.3°) were proinclined and the overbite (5.6 mm) had improved following surgery (Table 1).
| Table 1: Cephalometric values before and after premaxillary osteotomy. Statistical test Wilcoxon; significance, p < 0.05. | |||||
| T1 Median | SD | T2 Median | SD | Sig | |
| AGE (years) | 11.3 | 2,6 | 14.8 | 3,5 | 0.002 |
| CONVX (mm) | 8.4 | 6,4 | 2.2 | 5,3 | 0.002 |
| MXD (º) | 89.8 | 5,1 | 85.2 | 4,9 | 0.003 |
| FA (º) | 85.75 | 4,3 | 86.5 | 5,0 | NS |
| UP INC (º) | -4.5 | 12,1 | 17.8 | 20,7 | 0.003 |
| UPINC-FH (º) | 75.7 | 12,2 | 98.5 | 9,6 | 0.002 |
| UNDER (mm) | -1.5 | 7,7 | 1.62 | 7,0 | NS |
| OVER (mm) | 5.9 | 5,9 | 0.3 | 3,1 | 0.003 |
| ANB (º) | 6.7 | 4,5 | 2.5 | 4,3 | 0.003 |
| SNA (º) | 79.5 | 5,4 | 76.7 | 4,8 | NS |
| A-PT (mm) | 64.5 | 14,1 | 52.7 | 6,4 | 0.004 |
| A-FH (mm) | 37.7 | 6,4 | 33.6 | 4,9 | NS |
| B-PT (mm) | 48.8 | 16,3 | 44.1 | 10,6 | NS |
| B-FH (mm) | 83.6 | 12,1 | 79.6 | 6,1 | NS |
| ANS-PT (mm) | 68.6 | 14,9 | 56.7 | 5,9 | 0.004 |
| ANS-FH (mm) | 30.2 | 5,9 | 27.4 | 4,5 | 0.002 |
| UI-FH (mm) | 62.3 | 8,9 | 53.8 | 3,3 | 0.010 |
| UI-PT (mm) | 53 | 14,0 | 52.9 | 10,5 | NS |
| LI-PT (mm) | 55.6 | 14,3 | 50.2 | 9,8 | NS |
| UM-PT (mm) | 22.5 | 8,7 | 19.6 | 5,3 | NS |
| UM-FH (mm) | 51.8 | 7,5 | 49.8 | 5,2 | NS |
| LM-PT (mm) | 23 | 8,0 | 22.3 | 7,7 | NS |
| LM-FH (mm) | 54.5 | 8,8 | 51.7 | 5,0 | NS |
| PG-PT (mm) | 50.1 | 14,7 | 43.3 | 11,4 | 0.033 |
| PG-FH (mm) | 101 | 15,1 | 90 | 16,8 | NS |
In the model of the intragroup comparison of the intervention group (M1–M2 in study group), an increase in the intermolar width (4 mm) and intercanine width (7 mm), as well as intrusion of the upper incisor (4.43 mm), were observed after intervention (Table 2).
| Table 2: Analysis of models before and after premaxillary osteotomy. Statistical test Wilcoxon; significance, p < 0.05. | |||||
| M1 Median | SD | M2 Median | SD | SIG | |
| INTERM (mm) | 40.1 | 6,2 | 44,1 | 4,1 | NS |
| INTERC (mm) | 21 | 6,6 | 28 | 8,8 | NS |
| PERIM (mm) | 73.8 | 12,9 | 82,3 | 11,8 | NS |
| TOTAL MX H (mm) | 31.4 | 6,6 | 31,1 | 4,3 | 0.021 |
| ANT MX H (mm) | 9.5 | 4,6 | 8,7 | 2,4 | NS |
| EXTR UI (mm) | 4.6 | 1,2 | .17 | 3,2 | 0.002 |
When performing the intergroup comparison (study group before the intervention and control groups), values from the cephalometric analyses revealed that at point A (p = 0.033), the ANS (p = 0.024), and the upper molar (p = 0.038) were more diminished in the vertical plane in patients in the intervention group than those in the control group. In other words, the premaxilla and the upper molar were more extruded in those patients who were candidates for surgery (Table 3).
| Table 3: Cephalometric análisis of patients in the study group before osteotomy as compared to the control group. Mann-Whitney U test; significance, p < 0.05. | |||||
| T1 | SD | CONTROL | SD | SIG | |
| AGE (years) | 11.3 | 2,6 | 11.66 | 2,6 | NS |
| CONVX (º) | 8.4 | 6,4 | 6.7 | 4,1 | NS |
| MXD (º) | 89.8 | 5,1 | 89.87 | 3,8 | NS |
| FA (º) | 85.7 | 4,3 | 86 | 5,0 | NS |
| UP INC (º) | -4.5 | 12,1 | -.67 | 8,0 | NS |
| UPINC-FH (º) | 75.7 | 12,2 | 79.13 | 7,5 | NS |
| UNDER (mm) | -1.5 | 7,7 | -2.5 | 6,0 | NS |
| OVER (mm) | 5.9 | 5,9 | 1.7 | 4,4 | NS |
| ANB (º) | 6.7 | 4,5 | 5.9 | 3,1 | NS |
| SNA (º) | 79.5 | 5,4 | 78.8 | 4,0 | NS |
| A-PT (mm) | 64.5 | 14,1 | 59.1 | 5,3 | NS |
| A-FH (mm) | 37.7 | 6,4 | 31.5 | 7,2 | 0.033 |
| B-PT (mm) | 48.8 | 16,3 | 47 | 6,4 | NS |
| B-FH (mm) | 83.6 | 12,1 | 78.8 | 15,7 | NS |
| ANS-PT (mm) | 68.6 | 14,9 | 64.1 | 4,5 | NS |
| ANS-FH (mm) | 30.2 | 5,9 | 24.4 | 5,9 | 0.024 |
| UI-PT (mm) | 53 | 14,0 | 50.1 | 5,1 | NS |
| UI-FH (mm) | 62,3 | 8,9 | 54.8 | 11,4 | NS |
| LI-PT (mm) | 55.6 | 14,3 | 52.5 | 5,8 | NS |
| LI-FH (mm) | 56.5 | 7,9 | 53.4 | 12,8 | NS |
| UM-PT (mm) | 22.5 | 8,7 | 19 | 3,7 | NS |
| UM-FH (mm) | 51.8 | 7,5 | 45.5 | 8,6 | 0.038 |
| LM-PT (mm) | 23 | 8,0 | 20.3 | 4,8 | NS |
| LM-FH (mm) | 54.5 | 8,8 | 47.7 | 9,4 | NS |
| PG-PT (mm) | 50.1 | 14,7 | 45.9 | 7,3 | NS |
| PG-FH (mm) | 101 | 15,1 | 95.8 | 18,4 | NS |
In addition, the intergroup comparison revealed that patients in the control group before surgery had a lower total maxillary height (p = 0.021) and less extrusion of the upper incisor (p = 0.002). Thus, the premaxilla was more advanced, and the upper incisor more extruded, in patients who were determined to require premaxilla osteotomy (Table 4).
| Table 4: Analysis of models in patients in the study group before osteotomy as compared with the control group. Mann-Whitney U test; significance, p < 0.05 | |||||
| M1 Median | SD | CONTROL | SD | SIG | |
| INTERM (mm) | 40.1 | 6,2 | 40.2 | 5,1 | NS |
| INTERC (mm) | 21 | 6,6 | 24.5 | 4,4 | NS |
| PERIM (mm) | 73.8 | 12,9 | 71.5 | 6,9 | NS |
| TOTAL MX H (mm) | 31.4 | 6,6 | 24.4 | 2,6 | 0.021 |
| ANT MX H (mm) | 9.5 | 4,6 | 9.2 | 9,5 | NS |
| EXTR UI (mm) | 4.6 | 1,2 | 1.2 | 2,2 | 0.002 |
While multiple treatment protocols are available for patients with BCLP, our study is one of few that should a direct comparison between an intervention group and a non-intervention (control) group.
Premaxillary osteotomy is an intervention that presents difficulties in maintaining blood flow, and each case must be carefully evaluated for indication. Therefore, this study aims to evaluate the results after intervention and compare it with patients for whom the osteotomy was not indicated, to see if there is any variable that more clearly indicates those patients who are good candidates for premaxillary osteotomy.
In their bibliographic review of 16 articles on the management of premaxilla, Bittermann and colleagues (2016) found that most studies performed osteotomy and the alveolar graft during the same surgery [7]. In contrast to this, our protocol at the Hospital Sant Joan de Déu is performed in two surgeries, with osteotomy performed the first, followed a few months later by secondary alveolar bone grafting. These surgeries are performed separately to promote vascularization as well as to increase the stability of bone grafts. Osteotomy is recommended after 8 years of age, as performing it on patients younger than 6 years leads to more alterations in average facial growth [9].
Geraedts, et al. (2007) evaluated 40 patients with BCLP and performed a cephalometric analysis similar to ours, as they compared the intervention group with a control group without osteotomy [9]. They observed that after surgery, the intermaxillary relationship improves vertically and sagittally in the intervention group; these results are similar to that observed in our study. The Geraedtsm, et al. study concludes that osteotomy does not affect long-term maxillary growth [9]. Our study is of short duration, this line is the one we want to work on in future studies to see if these cases require less need for orthognathic surgery and to be able to evaluate the stability of the premaxilla.
Scott, et al. (2007) performed a study with 27 patients with BCLP who underwent osteotomy and 27 who did not require surgery [10]. After cephalometric evaluation before and after osteotomy, as well as later at 16 years of age, it was observed that the inclination of the upper incisors significantly increased after surgery in the intervention group; this is in line with the results of our study. Moreover, patients in the intervention group who underwent premaxillary osteotomy presented a lower requirement for orthognathic surgery and less time for orthodontic treatment [10].
Premaxillary osteotomy is a potentially good solution for severe forms of BCLP [17], as it is not possible to correct BCLP with orthodontics in these severe cases. There are few studies that quantify which patients need osteotomy and which patients do not, as differences in treatment protocols make comparison difficult. For example, in the protocol for treatment of vertical excess of the premaxillary, Meazzini, et al. (2010) suggests performing an orthopedic intrusion (Liou technique) in deciduous or mixed dentition and, specifically, an orthodontic intrusion in mixed dentition with a vertical excess < 7–8 mm and surgical intrusion in cases of mixed or permanent dentition with vertical excess > 7–8 mm [4].
According to our study, the values that differentiate a patient who requires osteotomy from one who does not is that the premaxilla is more extruded and more advanced, and the upper incisor and the upper molar are more extruded, in patients who are candidates for surgery. This can be used as a diagnostic tool for the indication of this type of surgery.
Improvements in the position of the premaxilla provided better functioning and aesthetics to the patient, as the premaxilla becomes positioned normally, the superior incisors have better pro-inclination and intrusion, and the overbite is improved; overall, this facilitates later orthodontic movements. These results are similar to those reported by Kyung, et al. (2016), who found that premaxillary repositioning effectively corrects a malpositioned premaxilla, achieving a successful restoration of maxillary arch coordination [17].
We did not find any other study that compares models of the maxillary arch before and after osteotomy. In our study, we observed an increase in intermolar and intercanine width, due to maxillary expansion prior to osteotomy. Maxillary expansion prior to osteotomy and alveolar grafts favor surgical access and increase the percentage of spontaneous canine eruption [13].
With this study we wanted to evaluate the dental and cephalometric changes after the premaxilla osteotomy performed, but above all we wanted to differentiate parameters that would help us to differentiate patients candidates for surgery from those who do not need it.
As objectives of future studies, we would increase the number of patients studied in the intervention group, objectify the requirements of long-term orthognathic surgery, and assess the stability of the position of the premaxilla throughout growth.
1. After osteotomy, patients present a premaxilla that is more normalized and level with respect to the occlusal plane, therefore giving a greater degree of inclination and intrusion of the upper incisors. This represents is a successful restoration of maxillary arch coordination.
2. Patients who need osteotomy present more extrusion and protrusion of the premaxilla and a greater extrusion of the upper incisors compared with those who do not.
3. Variables that differentiate patients who require osteotomy from those who do not can provide a diagnostic tool for determining when these type of surgeries are indicated.
The authors thank the Biostatistics Task Force of Joan Sentís for their valuable assistance with the statistical analyses; and Dr. Josep Rubio for his collaboration in this study.
- Rajmil L, Rivera A, Tobella L. Tratamiento ortodóncico en niños con malformaciones congénitas craneofaciales. Ministerio de Sanidad Servicios sociales e igualdad y la Agència de Qualitat i Avaluació Sanitàries de Catalunya. 2013.
- López Giménez A. Análisis de las alteraciones oclusales y morfología craneofacial del paciente fisurado: Universidad de Valencia. 2015.
- Koh K, Ki H, Suk Oh T, Man Kwon S, Woo Choi J. Treatment algorithm for bilateral alveolar cleft based on the position of the position of the premaxilla and the width of the alveolar gap. J Plast Reconstr Aesthet Surg 2013; 66: 1212-1218. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/23702194
- Meazzini MC, Lematti L, Mazzolemi F, Rabbiosi D, Bozzetti A, et al. Vertical excess of premaxilla in bilateral cleft lip and palate patients: A protocol for treatment. J Craniofac Surg. 2010; 21: 499-502. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/20489454
- Rolando Prada J, García A, Bermudez L, Rojas N, Martínez M, et al. Reposicionamiento de premaxilla protruida y conservación vascular en pacientes con LPH bilateral. Rev Colombiana de Cirugía Plástica y Reconstructiva. 2013; 19: 11-17.
- Jein-Wein Kiou E, Chen PKT, Huang CS, Chen YR. Orthopedic intrusion of premaxilla with distraction devices before alveolar bone grafting in patients with bilateral cleft lip and palate. Plast Reconstr. Surg. 2004; 113: 818- 826. PubMed: https://pubmed.ncbi.nlm.nih.gov/15108871
- Bittermann GK, de Ruiter AP,Janssen NG, Bittermann AJ, Van der Molen AM, van Es RJ, et al. Management of the premaxilla in the treatment of bilateral cleft of lip and palate: what can the literature tell us? Clin Oral Investig. 2016; 20: 207-217. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/26374747
- Aburezq H, Daskalogiannakis J, Forrest C. Management of the prominent premaxilla in BCLP. Cleft palate-Craniofacial J. 2006; 43: 92-95. PubMed: https://pubmed.ncbi.nlm.nih.gov/16405381/
- Geraedts W, Borstlap J, Groenewoud P, Stoelinga P. Long-term evaluation of BCLP patients after early secondary closure and premaxilla repositioning. Int J Oral Maxillofac Surg. 2007; 36: 788-796. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/17618084
- Scott J, Webb R, Flood T. Premaxillary osteotomy and guided tissue regeneration in secondary bone grafting in children with bilateral cleft lip and palate. Cleft Palate Craniofac J. 2007; 44: 469-475. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/17760480
- Toscano D, Baciliero U, Gracco A, Siciliani G. Long-term stability of alveolar bone grafts in cleft palate patients. Am J Orthod Dentofacial Orthop 2012; 142: 289-299. PubMed: https://pubmed.ncbi.nlm.nih.gov/22920694/
- Parri FJ, Soares-Oliveira M, García Aparicio L, Sancho MA, Sarget R, et al. Fisura labiopalatina bilateral: Experiencia de un centro con abordaje multidisciplinar. Cir Pediatr 2001; 14: 124-126.
- Li W, Liao L, Dai J, Zhong Y, Ren L, et al. Effective retropulsión and centralization of the severely malpositioned premaxilla in patients with bilateral cleft lip and palate: A novel modified presurgical nasoalveolar molding device with retraction screw. J Craniofac Surg 2014; 42: 1903-1908. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/25187377
- Kobayadhi S, Hirakawa T, Fukawa T, Maegawa J. Maxillary growth after maxillary protraction: Appliance in conjunction with presurgical orthopedics, gingivoperiosteoplaty, and Furlow palatoplasty for complete BCLP patients with protruded premaxilla. J Plas Reconstr Aesthet Surg. 2015; 68: 758-763. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/25778874
- Costanza Meazzini. Malformazioni craniofacciali: Coordinamento ortodontico-chirurgico. Ed Martina. 2011.
- Camporesi M, Baccetti T, Marinelli A, Defraia E, Franchi L. Maxillary dental anomalies in children with cleft lip and palate: a controlled study. Int J Paediatr Dent. 2010; 20: 442-450. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/20642471
- Koh KS, Han WY, Jeing WS, Oh TS, Kwon SM, et al. Premaxillary Repositioning in the Severe Form of Bilateral Cleft Lip and Palate. J Craniofac Surg 2016; 27: 1440-1444. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/27300460
- Rubio-Palau J, Prieto-Gundin A, Cazalla AA, et al. Three-dimensional planning in craniomaxillofacial surgery. Ann Maxillofac Surg. 2016; 6: 281–286. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/28299272
- Sierra NE, Ferrer-Fuertes A, Salazar-Perez B, Lee GYC, Martí-Pages C, et al. Surgical Repositioning of the Premaxilla Using a Minimally Invasive Endonasal Approach. Cleft Palate Craniofac J. 2018; 55: 830–836. PubMed: https://pubmed.ncbi.nlm.nih.gov/28140669